
CAN ENTROPY BE NEGATIVE FREE
Gibbs proposed that all spontaneous physical and chemical changes take place in the direction of a decrease in free energy. Gibbs free energy is the amount of energy left over after a chemical reaction has taken place. It is a measure of the stored energy in a reaction. ΔG = ΔH - TΔS, ΔG is change in the free energy, ΔH is the change in enthalpy, ΔS is the change in entropy and T is temperature in kelvin. Gibbs combined enthalpy change and entropy change by the following equation He proposed, that both change in enthalpy of a reaction and change in entropy of the reaction together decide if a reaction could be spontaneous. Here comes the great contribution of Gibbs. Therefore, a positive or negative change in entropy cannot predict if a reaction could be spontaneous, either. So, clearly, a spontaneous reaction is possible with both when entropy change is negative and also when entropy change is positive. But water goes from an ordered state of liquid to a disordered state of vapor. This is a typical example of a spontaneous reaction when the change in entropy is negative. The reason, as can be observed in the equation below, a mixture of solid and liquid reactants goes from a more disordered state to a completely solid phase product which is more ordered.ĬaO(s) + H2O(l) - > Ca(OH)2 (s), ΔS is negative. The entropy change ΔS of the reaction is negative. Reaction of lime with water takes place without any external help when lime is mixed with water. When lime is reacted with water, hydrated lime is produced.
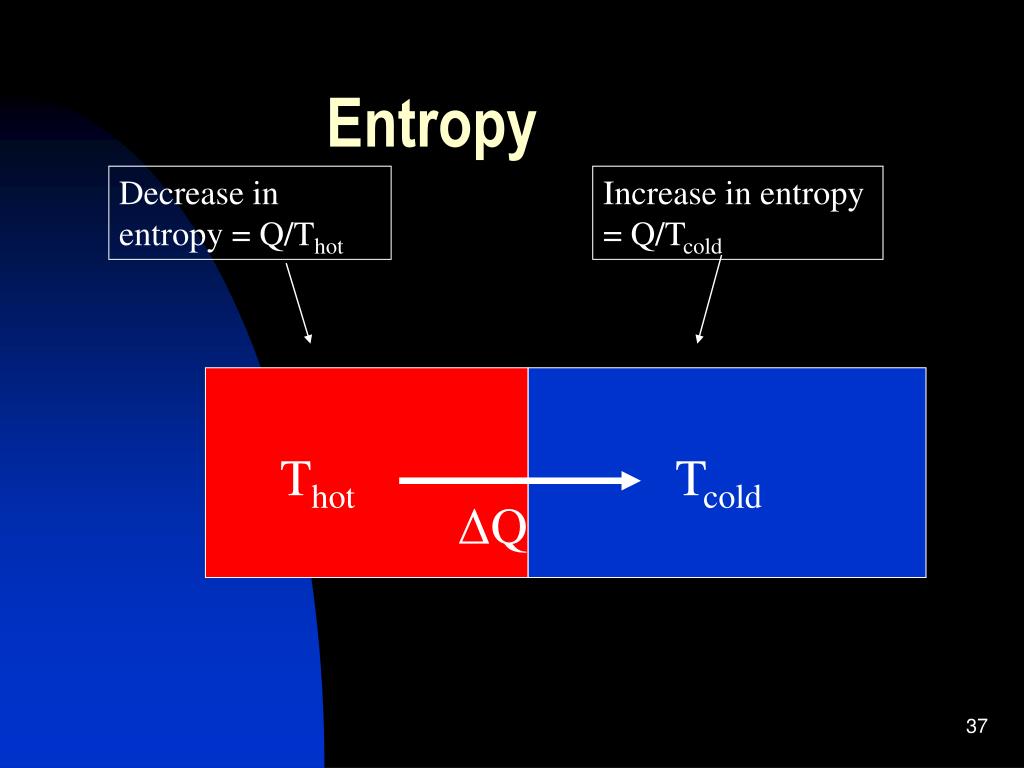
Now the question is, can we predict from the change in the entropy of a reaction between the products and reactants the spontaneity of a reaction? Let us take some examples of typical reactions. We have seen above that from the change in the enthalpy of a reaction, we cannot predict, if the reaction would be a spontaneous reaction. An increase in the degree of disorder of a system shows in a positive value of ΔS. In a gas, the molecules move completely independently of one another in a disordered manner, and the entropy of a gas is therefore always high.Įntropy is given the symbol S, standard entropy is expressed as S0 and change in entropy as ΔS. When it melts, the ions are free to move, the solid moves to a state of disorder, the entropy increases. Let us take some examples, a crystalline solid, with regular arrangement of ions, has low entropy. The degree of disorder or randomness in a system is measured by a physical quantity called entropy. The common factor in all these four cases of spontaneous reactions is a transition of the system from an ordered arrangement of particles to less ordered arrangement. In the fourth case, when water evaporates, the association of water molecules is replaced by individual water vapor molecules moving independently in vapor phase with much higher kinetic energy. In the third case, when ice melts, the regular hydrogen bonded structure of ice is replaced by less associated fluid which is water.
In the second case, when potassium chloride dissolves, the regular arrangement of the crystal structure is replaced by random distribution of mobile ions in solution. When there is combustion of methane and the reaction generates heat there is significant increase of kinetic energy of the product molecules of carbon dioxide and steam, which makes the individual gas molecules moving rapidly in all directions. If we look at all four reactions above, there is a common factor. Question: What makes a reaction spontaneous? Therefore, any enthalpy change in a particular direction, positive or negative, cannot be the only factor to decide if a chemical reaction would take place? There must be some additional factors. We saw in the above reactions that both exothermic and endothermic reactions could be spontaneous. Essentially, it depends on the difference in enthalpy between the products and reactants of a reaction. H2O(s) - > H2O(l), ΔcHo = 6.0 kj / moleĪbove examples show, both exothermic and endothermic reactions could be spontaneous.Įnthalpy (H) is a measure of how much energy is released or absorbed during a chemical reaction. KCl(s) + aq - > KCl(aq), ΔcHo = 19 kj / mole

Similarly there are spontaneous reactions which are endothermic, like It is an exothermic reaction and it is a spontaneous reaction.

When ΔG is negative, the reaction would go in the direction of reactants forming products.Ī reaction is called spontaneous when it takes place of its own accord without any external help.ĬH4(g) + 2 O2(g) - > CO2(g) + 2 H2O(g), ΔcHo = −891 kj / mole A spontaneous reaction may involve an increase or decrease in enthalpy, it may involve an increase or decrease in entropy, but it will always involve a decrease in free energy that is a negative ΔG. All spontaneous physical and chemical changes take place in the direction of a decrease in free energy.


 0 kommentar(er)
0 kommentar(er)
